Have you ever wondered what makes glaciers retreat, metals reshape, and chocolate bars yield to the warmth of your touch? It’s the captivating phenomenon of melting, and it’s at the heart of this article.
So, what are the things that melt? At a glance, ice, chocolate, butter, wax, sugar and this list extends to precious metals like gold and everyday materials like plastic and rubber.
These things melt due to the application of heat, which increases their internal energy, causing their molecular structure to transition from a solid to a liquid state.
What Does the Word ‘Melt’ Mean?
The word “melt” refers to the process in which a substance changes from a solid state to a liquid state as a result of heating or an increase in temperature.
It involves the breaking of intermolecular bonds or forces that hold the substance’s particles together in a solid structure, allowing them to move more freely and take on a liquid form.
The word “melt” is also used metaphorically to describe the softening or dissolving of something, such as one’s heart “melting” due to an emotional experience.
Whether you’re a curious explorer of the natural world or a practical expert in various fields, this article promises to enthrall you.
A Visual List of Things That Melt
Here’s a list of various substances and materials that can melt under specific conditions:
Ice

Ice is a solid form of water that melts at 32°F (0°C). This transformation occurs because heat energy breaks the bonds holding the water molecules together in a crystalline structure, allowing them to move more freely and transition from a solid to a liquid state.
Ice’s ability to melt at relatively low temperatures is why it’s essential for cooling drinks and preserving food.
Its transformation from solid to liquid plays a fundamental role in our daily lives and in the Earth’s natural processes, like the melting of polar ice caps due to rising temperatures.
Butter

Butter is a solid at lower temperatures and melts at approximately 90-95°F (32-35°C). The process of butter melting is a result of its composition, which consists of fats and water.
As it heats, the fat molecules in butter break down and become a liquid, while the water content turns into steam.
This transformation is why butter transitions from a solid to a golden, creamy liquid when exposed to heat.
Chocolate

Chocolate, a beloved treat, undergoes a delicious transformation when it melts. Its melting point varies depending on the type but generally falls around 90°F (32°C).
This is because chocolate contains cocoa butter, a fat that has a relatively low melting point.
When exposed to heat, the cocoa butter in chocolate becomes more fluid, causing the solid chocolate to turn into a luscious, glossy liquid.
Wax
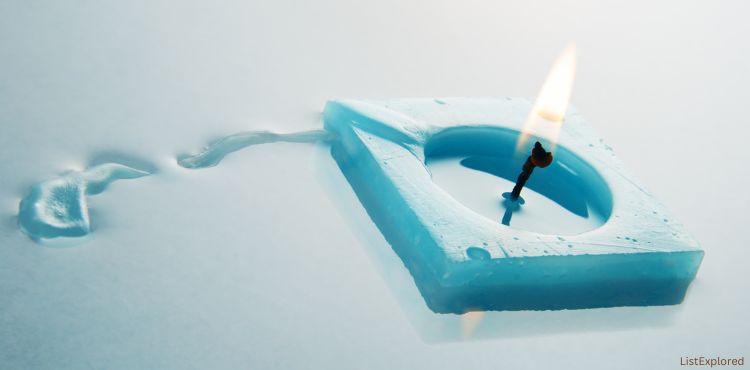
Wax, a versatile substance, has a wide range of melting points depending on its composition and intended use.
Common paraffin wax, often used in candles, melts at approximately 99-145°F (37-63°C). Beeswax, another natural wax, has a higher melting point, around 144-147°F (62-64°C).
When heat is applied, the solid wax undergoes a phase change, transitioning from a solid to a liquid state.
This transformation is key to its use in candles, crayons, and other products.
Ice Cream

Ice cream is a beloved dessert. Generally, it begins to melt when encounters temperatures above its freezing point. It typically begins to melt at temperatures above 32°F (0°C).
The main reason for ice cream’s melting is its high sugar and fat content, which lowers its melting point.
As the temperature rises, the solid ice cream starts to turn into a creamy, velvety liquid.
This mouthwatering process is especially evident on a sunny day or when served alongside a warm dessert.
Sugar

Sugar, a versatile sweetener, begins to melt around 366°F (186°C). When heated, sugar crystals break down and transition from a solid to a liquid form.
This process, known as caramelization, transforms sugar into a rich, golden-brown liquid that’s used in a variety of culinary applications.
This liquid sugar is used to create a wide range of sweet delights, from caramel candies to crème brûlée.
The process of melting sugar is not only a culinary marvel but also a sensory experience, as it releases a delightful combination of flavors and aromas.
Cheese

Cheese is a delectable dairy product and the melting point of cheese varies depending on the type.
For example, mozzarella cheese starts to melt at around 130-140°F (54-60°C), making it the perfect choice for pizza.
Cheddar cheese, another popular variety, melts at a slightly higher temperature of about 150-170°F (65-77°C).
As cheese is heated, its proteins, fats, and water content interact, leading to a phase change from a solid to a gooey, liquid state.
This property makes cheese a favorite for creating creamy sauces, gooey sandwiches, and countless other savory dishes that delight our taste buds.
Snow
Snow is a natural wonder that transforms landscapes into winter wonderlands, and begins to melt at temperatures above its freezing point.
Typically, snowflakes start to melt at around 32°F (0°C). This process occurs as the heat from the environment or the sun’s rays is absorbed by the snow, causing the delicate ice crystals to transition from their solid form to liquid water.
Salt
Salt is a common mineral used in various culinary and industrial applications, doesn’t actually melt in the traditional sense, like many other materials.
Instead, it undergoes a transformation known as dissolution when it comes into contact with water.
Salt dissolves into water at temperatures below its boiling point, which is around 2,000°F (1,093°C).
This dissolving process occurs as the salt crystals break apart into individual ions, creating a saline solution.
Understanding the dissolution of salt is essential for seasoning dishes, preserving food, and facilitating chemical reactions in various industries
Rubber
Rubber, a versatile material known for its elasticity and resilience, undergoes an interesting transformation when exposed to heat.
It starts to melt or soften around 392°F (200°C). As heat is applied, the polymer chains in rubber begin to lose their ordered structure and become more flexible, leading to a transition from a solid to a rubbery, semi-liquid state.
This property makes rubber suitable for a wide range of applications, including tires, rubber bands, gaskets, and countless other products.
Candy
Candy, those sweet confections that delight our taste buds, can exhibit a wide range of melting points depending on their ingredients.
For example, hard candies, like lollipops, begin to melt at relatively low temperatures, around 160-190°F (71-88°C), making them a tasty, melty treat.
On the other hand, chocolate candies, like truffles or chocolate bars, start to melt at about 90°F (32°C) due to the low melting point of cocoa butter.
When heat is applied, the sugar and fats in candies become more fluid, resulting in the delectable, gooey texture that we love.
Crayons
Crayons, the beloved coloring tools of children and artists alike, have a relatively low melting point. These waxy coloring implements start to melt at around 105°F (40°C).
When exposed to heat above this temperature, crayons undergo a transformation from a solid, colorful stick to a soft, vibrant, and blendable liquid.
Plastic
Plastic is a ubiquitous material in our modern world, and undergoes a transformation from a solid to a liquid state when exposed to heat.
The specific melting point of plastic varies depending on its type and composition, but it typically falls within the range of 250°F to 500°F (121°C to 260°C).
This transition occurs as the heat provides enough energy to weaken the bonds between polymer chains, allowing them to flow and take on a more fluid form.
Glass
Glasses often seen as a solid material, possess a unique behavior when it comes to melting.
Unlike most solids, glass doesn’t have a specific melting point. Instead, it gradually softens and transforms into a liquid as it’s heated.
This process is known as “softening” rather than melting, and it occurs over a range of temperatures, typically starting around 1,700-2,200°F (927-1,204°C), depending on the type of glass.
Metals That Melt
Metals are known for their wide range of melting points. Some have high melting points, while others melt at relatively low temperatures. Here’s a list of metals, each with its respective melting point:
- Lead: Lead is a chemical element with the symbol Pb and atomic number 82. It is a heavy metal known for its toxicity, but it also has several industrial and historical applications. It begins to melt at a temperature of about 621°F (327°C). This low melting point has made lead a valuable material in various applications throughout history, including plumbing, batteries, and as a component in alloys.
- Iron: Iron, a fundamental element with remarkable properties, has a melting point of approximately 2,800°F (1,538°C). This high melting point is a result of iron’s strong metallic bonds, which require significant heat energy to break. When exposed to the intense heat necessary for melting, iron transitions from a solid to a red-hot, molten state, allowing it to be molded and shaped into various structures and products.
- Copper: Copper is a metal known for its excellent electrical conductivity, and undergoes a phase change from solid to liquid at a relatively high melting point of around 1,984°F (1,085°C). This property is vital for its widespread use in electrical wiring, plumbing, and various industrial applications. When exposed to intense heat, copper transforms from a solid, ductile material into a fiery, molten liquid.
- Aluminum: Aluminum, a lightweight and versatile metal, begins its transformation from a solid to a liquid state at approximately 1,221°F (660°C). This relatively low melting point compared to other metals is a key factor in aluminum’s widespread use in various industries, from aerospace to beverage cans.
- Gold: Gold, symbolized as Au from the Latin “aurum,” is a chemical element with atomic number 79. It’s a precious metal synonymous with wealth and luxury, has a relatively high melting point of around 1,948°F (1,064°C). This characteristic is essential for its use in crafting exquisite jewelry and intricate ornaments. When subjected to the right level of heat, solid gold transforms into a radiant, shimmering liquid, allowing artisans to create intricate designs and fine details.
- Silver: Silver, another precious metal renowned for its lustrous beauty, boasts a lower melting point compared to gold. It begins to melt at around 1,763°F (961°C). This characteristic makes silver a valuable material in jewelry, silverware, and various industrial applications.
- Tin: Tin, a malleable and versatile metal, has a relatively low melting point of approximately 449°F (232°C). This property makes tin a valuable material in various industries, especially in the production of tin cans, solder, and alloys. When subjected to heat, solid tin transforms into a silvery, molten liquid that flows easily, filling gaps and forming strong connections.
- Mercury: Mercury, the only metal that remains in liquid form at room temperature, is known for its remarkably low melting point. It begins to melt at a chilling -37.89°F (-38.83°C) and transitions into a shimmering, silvery liquid.
- Tantalum: Tantalum, a rare and corrosion-resistant metal, boasts an exceptionally high melting point. It begins to melt at an impressive 5,463°F (3,017°C). This remarkable property is a key reason for its use in various high-temperature applications, such as in the aerospace and electronics industries.
- Tungsten: Tungsten, often referred to as the “heavy metal,” boasts an exceptionally high melting point. It begins to melt at a scorching 6,192°F (3,422°C). This remarkable property has earned it the reputation as one of the most heat-resistant materials known to humanity.
- Rhenium: Rhenium, one of the rarest elements on Earth, is celebrated for its extraordinarily high melting point. It begins to melt at a staggering 5,766°F (3,180°C). Rhenium’s remarkable heat resistance is crucial in applications such as aerospace, electronics, and the production of superalloys.
- Bismuth: Bismuth, a distinctive and colorful element, has a relatively low melting point, making it one of the few elements that expand when they freeze. It begins to melt at a mere 520°F (271°C). The low melting point of bismuth has also made it an essential component in fire sprinkler systems, as it can melt when exposed to a fire, triggering the release of water to suppress the flames.
- Gallium: It’s an unusual metal with fascinating properties and has a remarkably low melting point of approximately 85.6°F (29.8°C). This property makes gallium a captivating element, as it remains solid at room temperature but turns into a shiny, silver liquid when held in your hand.
- Cobalt: Cobalt, a transition metal known for its brilliant blue hue, has a relatively high melting point. It begins to melt at around 2,723°F (1,495°C). This property makes cobalt valuable in various industrial applications, such as the production of high-strength alloys for aerospace and medical equipment.
- Francium: Francium, a highly radioactive and extremely rare alkali metal, has a very low melting point compared to most other metals. It begins to melt at just above 80°F (27°C). However, due to its extreme radioactivity and scarcity, it is not typically observed in a pure, solid state. As a result of its low melting point and highly reactive nature, francium is primarily studied through its radioactive decay and is not used in practical applications.
- Indium: Indium, a soft and malleable metal, has a relatively low melting point, which adds to its versatility. It starts to melt at approximately 313.9°F (157.7°C). When exposed to moderate heat, solid indium transforms into a silvery, molten liquid, ideal for creating soldering materials and forming tight connections between electronic components.
- Hafnium: Hafnium is a chemical element with the symbol Hf and atomic number 72. It is a transition metal that shares several similarities with zirconium, and the two elements are often found together in nature. It is a refractory metal with a very high melting point and begins to melt at an impressive 4,048°F (2,230°C). It is often used in nuclear reactors and high-temperature applications.
- Neptunium: Neptunium, a radioactive and artificial element, possesses a relatively low melting point. It begins to melt at around 1,191°F (644°C). Due to its radioactive nature and scarcity, neptunium is primarily studied for scientific research and nuclear applications.
- Osmium: Osmium is one of the densest elements. Osmium is a chemical element with the symbol Os and atomic number 76. It is one of the platinum group metals, which also includes platinum, palladium, rhodium, ruthenium, and iridium. It begins to melt at an incredibly high temperature of 5,491°F (3,033°C).
- Iridium: Iridium, a dense and corrosion-resistant metal, possesses a very high melting point. It begins to melt at around 4,435°F (2,466°C). This exceptional property makes iridium invaluable in industries where materials must withstand extreme heat.
- Nickel: Nickel is a chemical element with the symbol Ni and atomic number 28. It is a metallic element that is known for its silvery-white appearance and various useful properties. It begins to melt at approximately 2,651°F (1,455°C). Nickel is valuable in various industrial applications, such as in the production of stainless steel and nickel-based alloys.
- Zinc: Zinc is a chemical element with the symbol Zn and atomic number 30. It’s a commonly used metal with numerous applications, has a moderate melting point. It begins to melt at approximately 787°F (419°C). It is a metallic element that plays important roles in various industrial, biological, and environmental processes.
- Molybdenum: Molybdenum, a refractory metal prized for its high melting point and strength, begins to melt at an impressive 4,748°F (2,623°C). When subjected to intense heat, solid molybdenum transforms into a molten, silvery liquid, all while maintaining its structural integrity.
- Platinum: Platinum is a chemical element with the symbol Pt and atomic number 78. It begins to melt at around 3,215°F (1,769°C). It is a precious and rare metal known for its lustrous, silver-white appearance and exceptional chemical and physical properties.
- Protactinium: Protactinium is a chemical element with the symbol Pa and atomic number 91. It is a radioactive metal and is part of the actinide series on the periodic table. In its pure form, protactinium is a silvery-gray metal that is solid at room temperature. It has a relatively high melting point of 1,569 degrees Celsius (2,876 degrees Fahrenheit) and a boiling point of around 4,027 degrees Celsius (7,280 degrees Fahrenheit).
Some Facts About Things That Melt
- Amorphous Solids: Some materials do not have a distinct melting point but gradually transition from a solid to a liquid over a range of temperatures.
- Supercooling: Some liquids can be supercooled, meaning they remain in a liquid state below their normal freezing point.
- Triple Point: Each substance has a specific temperature and pressure at which it can exist in all three states simultaneously: solid, liquid, and gas. This point is called the triple point.
- Melting Glaciers: As global temperatures rise, glaciers and polar ice caps are melting at an accelerated rate, contributing to rising sea levels and impacting ecosystems worldwide.
- Melting Point Depression: Adding impurities to a substance can lower its melting point. This is the principle behind salting icy roads; the salt lowers the freezing point of water, preventing ice formation.
Final Words
In the end, “things that melt” not only teach us about the properties of matter but also inspire us to adapt, change, and embrace transformation, whether in the laboratory or in our daily lives.
I promise to continually update this article as I find new things that melt. Did I leave anything behind? Please, let me know in the comments.
Remember, sharing is caring….!!!
You may also like:
